CAP Sampling & Testing Programme
These activities involve the EU/EEA OMCL Network only.
Background
Since 1995, the Commission of the European Union has being granting community marketing authorisations for new medicines for both human and veterinary use. These are known as centrally authorised products (CAP). Such products can be marketed in all EU/EEA member states, so a co-ordinated approach to controlling the quality of these products is necessary. In June 1999, a contract governing an annual CAP Sampling & Testing Programme was signed by the European Medicines Agency (EMA) and the EDQM. The EMA is the sponsor of the programme and has overall responsibility for it, whereas the EDQM co-ordinates the sampling and testing operations. The EDQM’s duties include reporting the results of the testing programme and proposing follow-up actions, if necessary, to the EMA. National inspection services gather sample products from the market and members of the EU/EEA OMCL Network test them.
Since 2009, each annual programme has included products selected using a risk-based approach. Previously, a systematic approach had been used, i.e. each yearly programme included products that had been granted a community market authorisation 3 years previously.
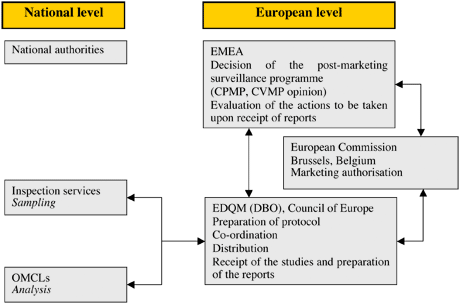
The Procedure
The list of products to be included in the annual programme is prepared by the EMA Secretariat in collaboration with the EMA Scientific Committees. This is sent to the EDQM, which then co-ordinates the sampling and testing operations on the basis of the information provided by the marketing authorisation holders (MAH) at the request of the EMA. Samples are collected, in principle, from throughout the entire medicines distribution chain (i.e. from wholesalers and community and hospital pharmacies) by nominated national sampling contacts. Samples are collected, on average, from three different countries. These are sent to the EDQM, which allocates them to national control laboratories for testing in accordance with well-established protocols derived from marketing authorisation (MA) dossiers. The EDQM collects the analyses and results and produces a report, which includes the quality control results and proposals for follow-up action, if necessary. This report is sent to the EMA.
Benefits of the CAP Sampling & Testing Programme
A position paper: The advantages and benefits of the CAP Surveillance Project, was released in November 2013. This document underlines the value of this multi-disciplinary activity and also includes a statistical evaluation of the programme as well as a chapter discussing future considerations.
Achievements in 2024
The concerted Centrally Authorised Products (CAP) Sampling & Testing programme has entered its 26th consecutive year. Since its initiation, the programme – originally covering ‘regular’ medicinal products for human and veterinary use and generics – has been continuously improved and further extended thanks to the close collaboration between its partners. Two additional CAP testing activities had been added in 2019.
A list of products to be included in the annual Regular programme is jointly prepared by the EMA Secretariat and the EMA Scientific Committees, with input from the OMCL Network and the EDQM, applying a risk-based approach. For the Generics programme, three testing campaigns are conducted every year.
The 2024 CAP Regular programme included 36 medicinal products for human use (19 biologicals, including three insulin-based products, and 17 chemical products) and seven medicinal products for veterinary use (three immunobiological products and four chemical products). One active pharmaceutical ingredient (API) testing project was included in the 2024 programme, as well.
The 2024 CAP Generics surveillance programme covered products containing Lenalidomide, Meloxicam and Zoledronic acid.
In response to the increasing number of biosimilars, Adalimumab was selected as the next project in 2024 under the Biosimilar programme as a continuation of the first three projects covering the molecules Filgrastim, Etanercept and Rituximab.
The Parallel distributed products testing programme was set up to verify the authenticity of the products selected. Seven products were tested under this programme in 2024.
The results of the 2024 programmes showed that the vast majority of products tested were of the expected quality and complied with the authorised specifications. By cut-off date 31 December 2024, no product with out-of-specification results has been found. However, regulatory, technical or editorial findings for a few products have been reported to and followed up by the EMA.
CAP Procedure
(Click on the thumbnail below to enlarge it)
General procedure for S&T CAP regular programme :
- PA/PH/CAP (05) 49 R13 (valid until finalisation of the 2024 CAP programme)
- PA/PH/CAP (05) 49 R14 (only valid as for the 2025 CAP Programmes)
General procedure for the Generics CAP S & T programme: In effect from January 2025:
General procedure for the Biosimilar CAP S & T programme: In effect from January 2025:
General procedure for the Parallel Distribution CAP S & T programme: In effect from December 2018: