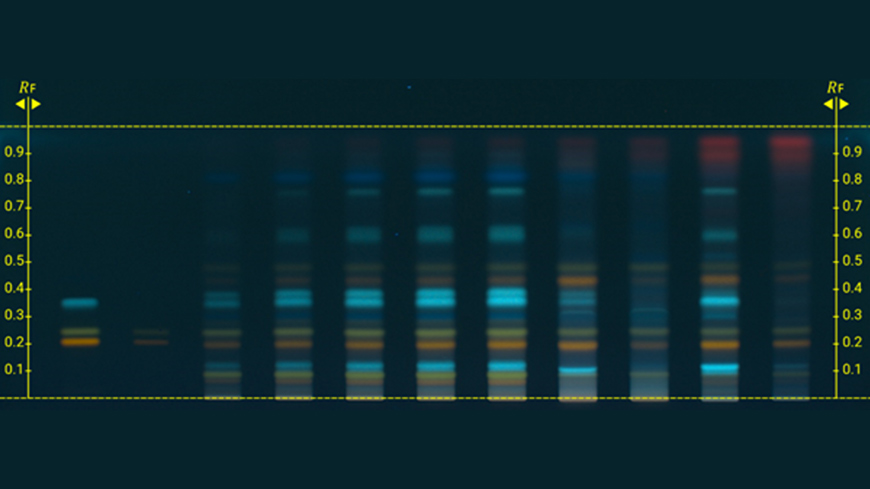
The first two monographs, Calendula for homoeopathic preparations (2492) and Chamomilla for homoeopathic preparations (2493), resulting from the pilot study on the use of semi-quantitative high-performance thin-layer chromatography (HPTLC) fingerprinting for homoeopathic preparations containing non-toxic compounds have just been published in Pharmeuropa 36.2 for comment.
The European Pharmacopoeia Commission decided to launch the pilot study in 2019 with a view to harmonising current practices in Europe. HPTLC provides a semi-quantitative evaluation of levels of defined markers as a quality control in monographs on homoeopathic preparations. The method consists in evaluating the intensity of the spot due to the marker in the test solution and comparing it with the intensity of the same spot in a reference solution containing the marker at a known concentration. Marker levels are then evaluated by visual inspection or using appropriate software.
The deadline for comments is 30 June 2024 and, of course, the EDQM welcomes comments from all interested parties.
One other monograph (Arnica planta tota for homoeopathic preparations (2580)) is also part of this pilot study and will be published in a future issue of Pharmeuropa.