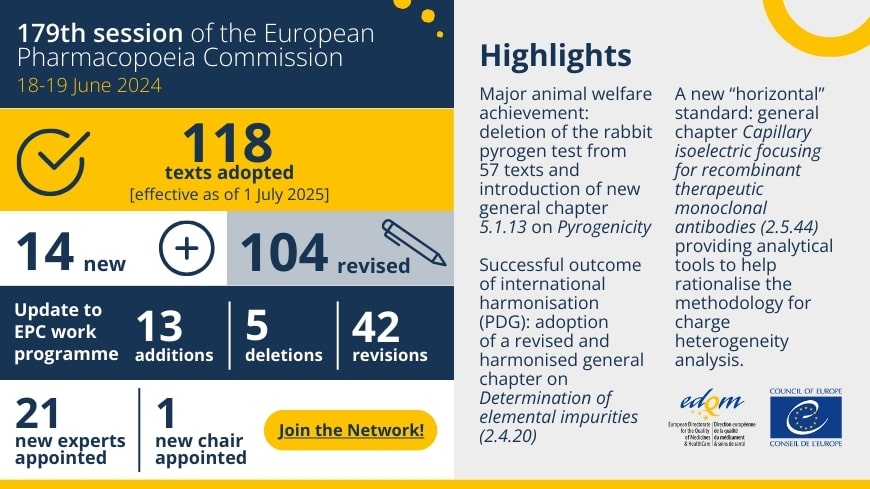
Pyrogen detection is essential for ensuring the safety of parenteral medicines. For decades, the rabbit pyrogen test (RPT) has been the traditional method.
The RPT involves measuring the rise in body temperature in rabbits following intravenous injection of the substance to be examined.
Despite multiple efforts to encourage medicine developers to move away from the RPT, the test is still widely used to detect pyrogenic substances, consuming a large number of rabbits worldwide.
At its 179th session in June 2024, as the outcome of a broad exercise aiming at the complete removal of the RPT from the European Pharmacopoeia (Ph. Eur.), the Ph. Eur. Commission adopted 57 revised texts from which the RPT has been deleted, together with a new general chapter on Pyrogenicity (5.1.13), marking the end of the RPT era in the Ph. Eur. A list of these texts can be found here.
This is a major achievement for animal welfare and for the advancement of modern in vitro approaches for pyrogenicity testing. As a result, the use of the RPT will no longer be required in any text of the Ph. Eur. and it will be the responsibility of medicine developers to select a suitable in vitro test (e.g. the monocyte-activation test) to control the pyrogenicity of their product, based on a risk assessment as described in the new general chapter.
The revised texts omitting the RPT and Pyrogenicity (5.1.13) will be published in Supplement 11.8 of the Ph. Eur., with an implementation date of 1 July 2025.
Find out more in the press release "European Pharmacopoeia bids adieu to rabbit pyrogen test in its monographs".