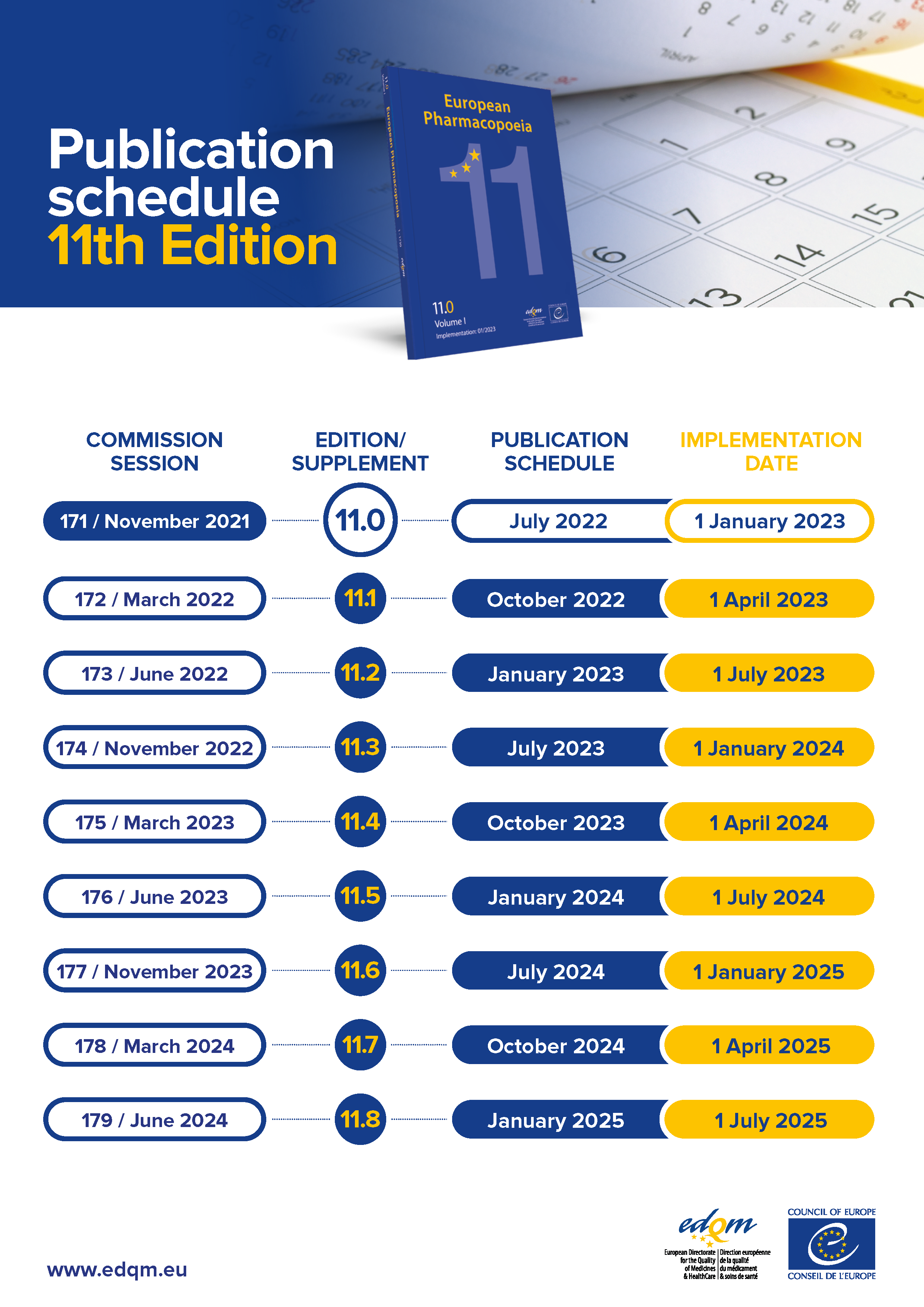
Available to order from July 2022 / Effective from 1 January 2023
The primary source for quality control standards
The European Pharmacopoeia (Ph. Eur.) is the primary source of official quality standards for medicines and their ingredients in Europe. Ph. Eur. standards provide a scientific basis for the quality control of a product throughout its life cycle, supporting the pharmaceutical industry and healthcare systems.
As laid down in the Council of Europe Convention on the Elaboration of a European Pharmacopoeia and in European Union and national pharmaceutical legislation, these standards are legally binding. They become mandatory on the same date in all states parties to the convention.
Learn more about the European Pharmacopoeia Commission
Join the community and participate in the elaboration and revision of its content.
Subscribe NOW and get the pharmacopoeia that delivers information for European markets before any other!
The 11th Edition key facts and figures
- New edition, legally binding in 39 European countries as of 1 January 2023 and applied in more than 130 countries worldwide.
- Continually updated and modernised to meet users' needs.
- The 11th Edition (including Supplement 11.8) of the Ph. Eur. contains:
- 2 528 monographs (including dosage forms);
- 397 general texts (including general monographs and methods of analysis);
- and about 2 930 descriptions of reagents.
Subscription formats
Online version: features and options
- fully cumulative versions, bilingual (English and French), with new features and direct access to complementary information (Knowledge database);
- tablet and smartphone friendly;
- compatible with recent versions of Windows, Linux and MacOS;
- direct access to Ph. Eur. online archives included for the duration of the subscription;
- featuring a new tool to toggle off change marks for improved readability and toggle on change marks for visibility of changes (for revised and corrected texts);
- other features: direct links to texts, search query management.
Note that with the 11th Edition, the EDQM will focus efforts on the online and print versions and will no longer provide a device version for activation on USB sticks or computers.
For more information and for technical specifications, please consult the technical requirements, the EDQM FAQs and the user manual.
Print version
- three initial volumes (11.0), followed by eight non-cumulative supplements (11.1 to 11.8);
- available in either English or French;
- now with QR codes for easier linking to the Knowledge database for each monograph and general chapter;
- contains a subscription key (EPID code) giving access to the Ph. Eur. online archives.
Note that the 11th Edition print version will no longer contain change marks in revised and corrected texts.
Prices and offers
- 570€ per annual subscription (print or online);
- a package including both print and online versions is available at a significantly reduced price;
- free shipping and handling is offered for orders placed online (print version);
- save money by purchasing more than one online licence at the same time;
- special price available for university online access.
For more information, consult the 11th Edition price list and see “Sales information” opposite.