Join the Network!
Have you ever considered sharing your expertise in a dynamic international network of scientists that makes a difference in the field of public health protection?
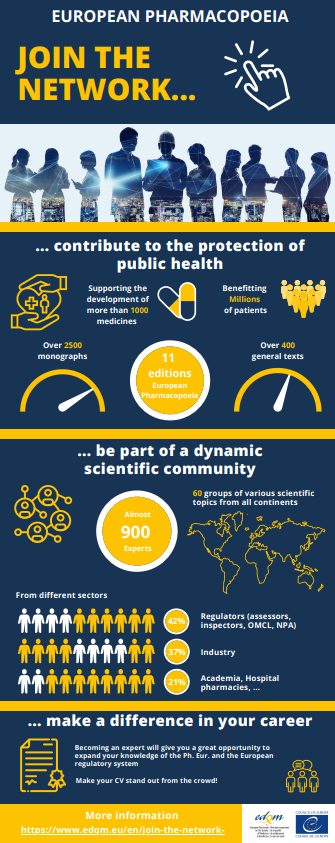
Would you welcome the opportunity to get to know other scientists from a wide variety of professional backgrounds and positively enhance your career development?
Then join the European Pharmacopoeia’s community of experts!
Why become a Ph. Eur. expert?
- Help shape Ph. Eur. texts at an early stage.
- Be part of and help build a network that brings together experts from a wide variety of professional backgrounds including experts from national authorities (e.g. pharmacopoeial authority, official medicines control laboratory, licensing authority, inspectorate), from the private sector (pharmaceutical or chemical industry), from academia or from a research organisation. This will give you a unique opportunity to share information and experience and to better understand difficulties linked to the elaboration and revision of pharmacopoeial texts, and to find a common way forward based on a mutual understanding.
- Network and exchange experiences in a European and international environment.
- Make your CV stand out from the crowd! Becoming an expert will give you a great opportunity to expand your knowledge of the Ph. Eur. and the European regulatory system.
How can I apply to become a member of a Group of Experts or a Working Party?
1. First check that your profile fulfils the criteria laid down under “Profile for experts” for the relevant group (see “Terms of reference and profile for members of Groups of Experts and Working Parties” document).
2. Complete:
- The Nomination form (available in English only)
- The Declaration of Interests form (available in English only)
3. Fill in these two forms and send them with an up-to-date Curriculum Vitae (please ensure that your expertise in the field relevant for the Group is evident when reading your CV).
Experts are appointed by the Ph. Eur. Commission. The EDQM will inform you of the Commission's decision usually in the month following the session at which your application is discussed.
Candidates from Ph. Eur. member states:
Applications are to be submitted to your national pharmacopoeia authority (NPA).
The terms of reference of all Ph. Eur. Groups of Experts and Working Parties are provided in the “Terms of reference and profile for members of Groups of Experts and Working Parties” document.
If you have any questions, please contact your national pharmacopoeia authority (NPA).
Candidates from non Ph. Eur. member states:
The Ph. Eur. welcomes input from experts from all around the world. Whether you are employed by a national authority, industry or academia, your contribution to preparing and maintaining Ph. Eur. texts will be highly valued.
The terms of reference of the Ph. Eur. Groups of Experts and Working Parties for which candidates from non Ph. Eur. member states may apply are provided in the “Terms of reference and profile for members of Groups of Experts and Working Parties” document.
Who better than the experts to talk about it?
- Rules of Procedure of the European Pharmacopoeia Commission
- Guide for the Work of the European Pharmacopoeia
- Code of Practice for the work of the European Pharmacopoeia
- Privacy statement of the European Pharmacopoeia
- Terms of reference and profile for members of Groups of Experts and Working Parties