CD-P-PH/PC - Pharmaceutical Practices and Pharmaceutical Care
"Appropriate use of medicines is key to ensuring the best clinical outcomes for patients"
“Medication is the most common intervention in health care and plays a vital role in maintaining our health and wellbeing. Evidence suggests that inappropriate use of medicines results in sub-optimal medication outcomes and significant health damage for patients, and it decreases the efficiency and effectiveness of health care systems. Since a long time, the Committee of Experts CD-P-PH/PC has, through a number of activities, initiatives and legal instruments, actively contributed to the development and implementation of quality and safety standards to advance the appropriate and safe use of medications, promote patient-centred care and ensure that patients obtain the best possible outcome from their medicine” - Dr Denis Gjika, Chair of the CD-P-PH/PC
Mission and work programme: setting quality and safety standards in pharmaceutical practices and pharmaceutical care
The Committee of Experts on Quality and Safety Standards in Pharmaceutical Practices and Pharmaceutical Care (CD-P-PH/PC) is overseen by the European Committee on Pharmaceuticals and Pharmaceutical Care (CD-P-PH) (steering committee) and is entrusted with improving pharmaceutical care and pharmaceutical practices in community and hospital pharmacy settings through specific programmes and policies. Its primary responsibilities, according to its terms of reference, are as follows:
- To develop and carry out a programme of activities aiming at improving public healthcare in Europe through promoting knowledge, skills, attitudes and values in care and practices involving pharmaceuticals. In particular, these activities comprise the development and promotion of guidance documents and recommendations on safe and good use of medicines, such as:
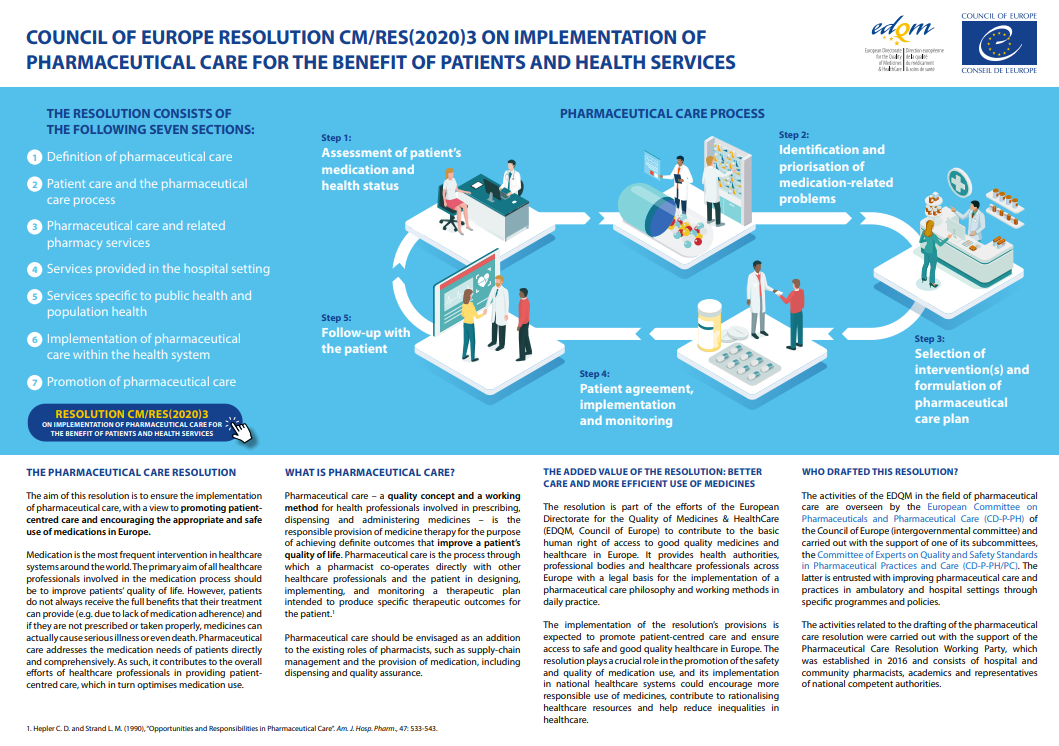
- Resolution CM/Res(2020)3 on the implementation of pharmaceutical care for the benefit of patients and health servicesGuidelines on medication review
- Guidance document on automated-dose dispensing
- Resolution CM/ResAP(2016)2 on good reconstitution practices in health care establishments for medicinal products for parenteral use
- Resolution CM/Res(2016)1 on quality and safety assurance requirements for medicinal products prepared in pharmacies for the special needs of patients
- To assist the CD-P-PH in monitoring the adequate implementation of the results of the relevant activities at national support in States Parties to the Convention on the Elaboration of a European Pharmacopoeia and assist the CD-P-PH in the evaluation and follow-up of the programme of activities mentioned above;
- to promote patient-centred care, improve interprofessional collaboration and advance appropriate use of medicines in hospital and community pharmacy settings;
- To maintain and develop links with national, European institutions and international organisations and professional bodies active in the fields of pharmaceutical practices and pharmaceutical care.
The CD-P-PH/PC consists of representatives from relevant public health authorities nominated by the governments of the States Parties to the Convention on the Elaboration of a European Pharmacopoeia. They include experts responsible for the preparation and follow-up of national policies in the field of pharmaceutical practices and pharmaceutical care.
View the Terms of Reference of the Committee of Experts CD-P-PH/PC.
26 January 2023
Council of Europe Resolution on the implementation of pharmaceutical care – A step forward in the promotion of appropriate use of medicines and patient-centred care
Register and receive the webinar recording HERE.
16 June 2021
Webinar on the Council of Europe Resolution on good reconstitution practices – a major contribution to the safety of patients receiving reconstituted medicines
Register and receive the webinar recording HERE.
26 November 2020
Webinar on the Council of Europe ‘Resolution on the implementation of pharmaceutical care’
Register and receive the webinar recording HERE.

The final report of the EDQM Pharmaceutical Care Indicators Project presents the results of the multinational validation study aimed at validating 4 basic sets of indicators to assess the quality of pharmaceutical care in Europe.
It also contains the data collection forms developed for and used in the validation study.
Activities under CD-P-PH (2018)